Excluded Volume of Polymer Chains
The ideal chain model neglects the interaction between the repeat units along the chain molecule, that is, it assumes that the volume that each repeat unit occupies does not affect the conformation of the polymer chain. This model works well for freely-jointed polymer chains (Kuhn molecules) in the melt but is not applicable to polymers in solution where the excluded volume (the volume that each repeat units occupies) has a significant effect on the overall conformation of the polymer chain. This situation is a very difficult mathematical and physical problem and of great importance to polymer science. Not surprising, many scientist have investigated the self-avoidance problem by means of analytical methods, such as perturbation theory or self-consistent-field theory. A good overview of the older models is given in Yamakawa's famous book "Modern Theory of Polymer Solutions"1. So far, there is no exact treatment of self-avoidance for long chain molecules. Thus, considerable work has been devoted to numerical methods such as exact enumeration, Monte Carlo or algebraic methods (recursion formula methods). A good overview of these methods has been given by Binder.2
A good starting point is a simple linear chain. This problem was first addressed by Flory3. We have chosen a slightly different approach since his formula somewhat overestimates the swelling of a polymer chain.
Let us assume that the polymer is in a state where its end-to-end distance R is close to its most probable value, lNα, where l is the (average) length of a repeat unit and N is the number of repeat units in the polymer chain. Let us further assume that the majority of the segments can be found in a spherical volume with a radius equal to R and that the solvent molecules are randomly distributed in this volume R3. These assumption are difficult to justify and one might argue that they lead to erroneous expressions. However, as Masao Doi4 and others have shown, the relations that can be derived from these assumptions describe, at least qualitatively, the approximate behavior of a self-avoiding polymer chain. The good agreement with experimental findings might be the compensation of errors.
Assuming a lattice model, there are R3/l3 lattice sites, where l3 is the volume of a cell. If we place the segments successively on these lattice sites, then the probability that a site is already occupied after i segments have been placed down is 1 - i l3/R3 and the probability that no overlap occurs in any chain configuration is given by
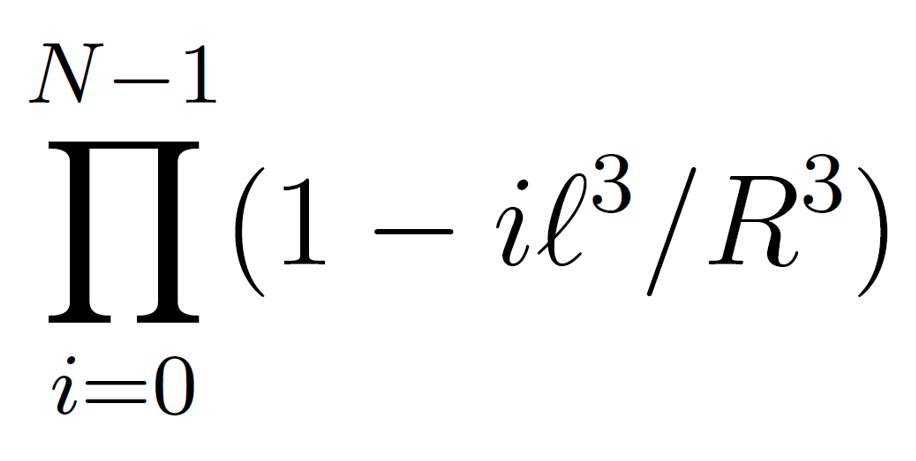
The probability W0(R) that an ideal chain has an end-to-end distance between R and R + dR is simply G(R,N) 4πR2 dR, where G(R,N) is the Gaussian probability distribution function of the end-to-end distance:
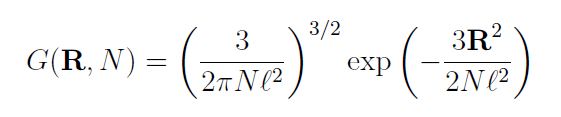
Therefore, the probability distribution W(R) of the end-to-end distance of a real chain is proportional to the product of the above described two factors:5
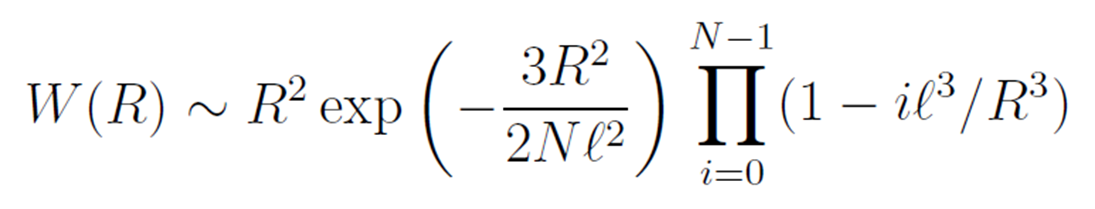
The various moments, like the mean square end-to-end distance <R2>, can be calculated from
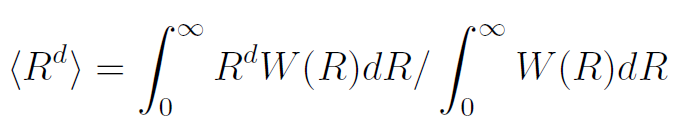
But this equation is difficult to solve. A good estimate for the size of the polymer chain is the value R that makes W a maximum (maximum term method). If the volume fraction of polymer segments in the sphere of radius R is small, then the approximation 1 - x ≈ e-x is valid and we obtain
![]()
Then
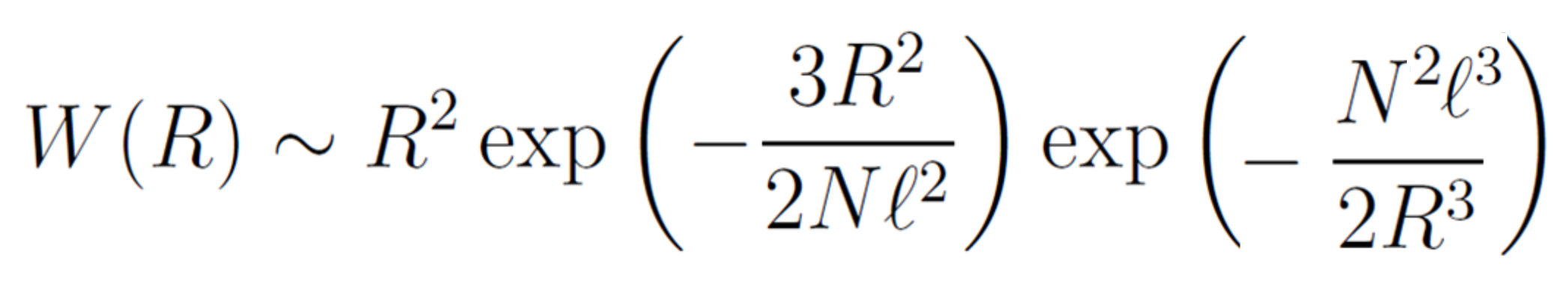
This equation describes the end-to-end probability distribution of a self-avoiding polymer chain. Both W and W0 have a maximum at a certain R value. The maximum of W0 occurs at R0* = (2Nl2/3)1/2 and the maximum of W can be found by differentiating the logarithm of the equation above. This gives
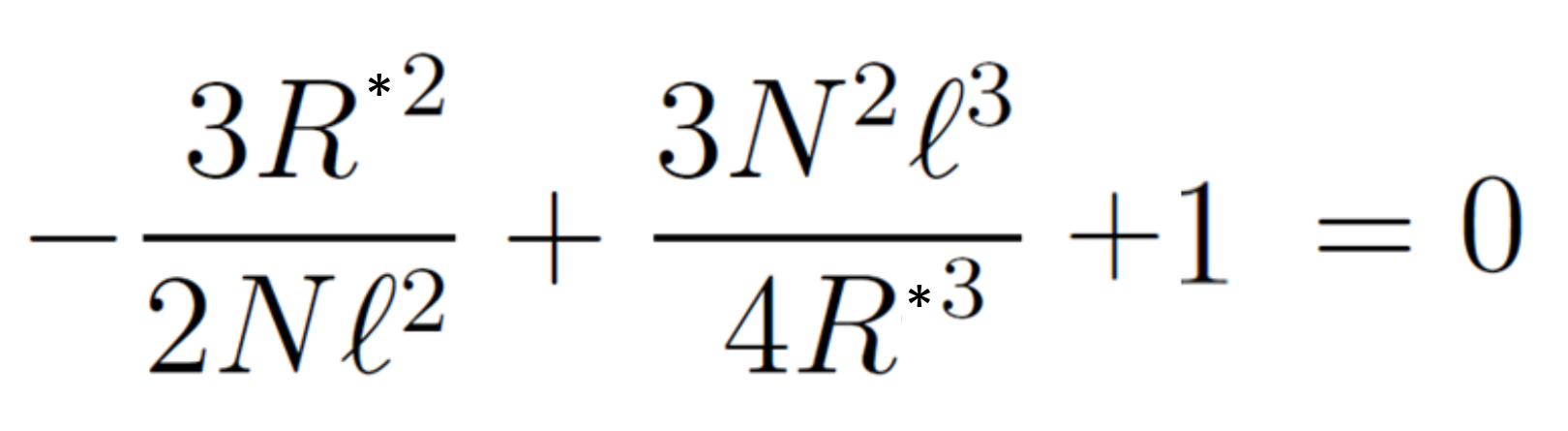
The first term describes the elastic deformation of the chain, and the second term is the excluded volume interaction. Solving this equation leads to the characteristic size of a self-avoiding polymer chain, R ≅ N3/5 = N0.6. The observed exponent is approximately 0.588.
References & Notes
H. Yamakawa, Modern Theory of Polymer Solutions, New York (1971)
K. Binder, Monte Carlo and Molecular Dynamics Simulations in Polymer Physics, Oxford University Press (1995)
P. J. Flory, J. Chem. Phys. 9, 660 (1941); 10, 51 (1942); 17, 303 (1949)
M. Doi, Introduction to Polymer Physics, Oxford Science Publications (1995)
This is only true for a polymer chain in an athermal solvent. It is well known that the size of a polymer coil depends on the type and temperature of the liquid in which the chain is placed. Due to the affinity with the solvent molecules, a polymer coil will expand (swell) when placed in a good solvent and contract when placed in a poor solvent.
Revised September 12, 2019